
- #SANGER SEQUENCE ANALYSIS UPDATE#
- #SANGER SEQUENCE ANALYSIS MANUAL#
- #SANGER SEQUENCE ANALYSIS VERIFICATION#
16, 17Ĭurrent Sanger sequencing automation generally supports the generation of NA sequences up to 800–1,000 bp.
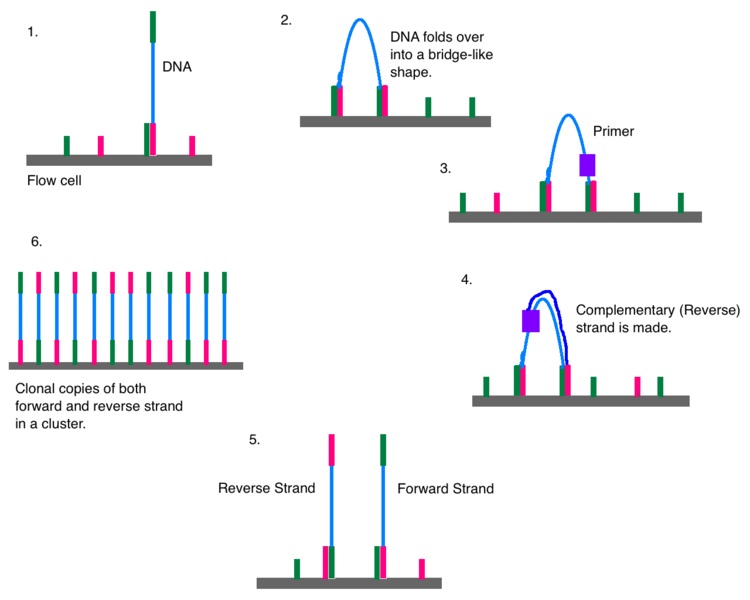
The inclusion of rate-limiting concentrations of the ddNTPs stops the elongation reaction as the ddNTPs are incorporated, resulting in distinguishable DNA fragments of various lengths. In the Sanger-sequencing approach, amplified DNA or complementary DNA (cDNA) is annealed to an oligonucleotide primer and then extended by the DNA polymerase enzyme that incorporates either a mixture of the 4 deoxynucleotide triphosphates (dNTPs: dATP, dGTP, dCTP, dTTP) or chain-terminating dideoxynucleotide triphosphates (ddNTPs: ddATP, ddGTP, ddCTP, ddTTP). 7, 10 Traditional Sanger sequencing not only forms the basis for the newer and automated approaches, but continues to be the most common sequencing approach used in VDLs for sequence verification, assay monitoring, and as the foundation for many phylogenetic analyses. Numerous modifications and advances in methodology have been published and adapted for use since sequence analysis was introduced in 1975, 16 including de novo sequencing and large-scale parallel sequencing (next-generation sequencing).
#SANGER SEQUENCE ANALYSIS MANUAL#
The focus of the guidance is on traditional Sanger sequencing, a technological approach that forms the basis for both manual and automated NA-sequencing approaches. Recognizing the critical importance of sequence analysis in current laboratory medicine, the Laboratory Technology Committee of the American Association of Veterinary Laboratory Diagnosticians (AAVLD) compiled the experience and utilized the expertise currently existing in accredited VDLs to provide consensus information and to propose national guidelines for sequence analysis, specifically targeting VDLs. This includes the responsibility for quality assessment of the sequencing results, manual editing of the generated sequence data as required, and final interpretation of the findings.Īs with any laboratory technique, the steps associated with sequence analysis, from sample preparation through final analysis of the results, require protocols and guidance describing the process and ensuring that the work is executed consistently. Regardless of whether outsourced or in-lab, sample preparation, analysis, and interpretation of sequencing results remain the responsibility of the submitting diagnostic laboratory. Logistically, for most VDLs, NA Sanger sequencing was routinely outsourced to specialized facilities and suppliers. Given the complexity and the relatively high cost of sequencing, sequence analysis has until recently remained a supplemental tool in most VDLs. In addition to serving as a confirmatory assay of high diagnostic specificity, high-quality NA sequence analysis is critical for initial molecular-assay development, monitoring the efficacy of molecular-based assays and their key components, forensic investigations into the source of an agent or disease outbreak, and for genotyping or differentiation between field and vaccine strains.
#SANGER SEQUENCE ANALYSIS VERIFICATION#
Sequence analysis is critical in laboratory medicine for the identification of emerging pathogens or new genotypes of existing pathogens, for identifying important evolutionary changes in the genomes of recognized pathogens, and critically, for verification of unusual laboratory findings such as recovering a specific pathogen from a new species or a new geographic area. Throughout this evolution, and continuing today, Sanger DNA sequencing has served as the gold standard for determination of nucleic acid (NA) sequences, whether occurring naturally or produced synthetically. The trend in molecular approaches in diagnostic medicine has evolved from selective use of standard PCR to routine and widespread use of real-time PCR (rtPCR), and most recently to bench-level next-generation, or high-throughput, sequencing.
#SANGER SEQUENCE ANALYSIS UPDATE#
NGS can identify large chromosomal rearrangements down to single nucleotide variants.It is a critical function of veterinary diagnostic laboratories (VDLs) to continually update testing methodologies in order to provide the best, most accurate, and cost-effective testing for their clients. † Mutation resolution is the size of the mutation identified. * Discovery power is the ability to identify novel variants. Time-consuming for sequencing low numbers of targets (1–20 targets).Less cost-effective for sequencing low numbers of targets (1–20 targets).Low scalability due to increasing sample input requirements.Not as cost-effective for high numbers of targets (> 20 targets).More data produced with the same amount of input DNA ‡.Higher sequencing depth enables higher sensitivity (down to 1%).



 0 kommentar(er)
0 kommentar(er)
